
3.2 Addition reactions with unsaturated carbon compoundsĮlectronic properties Hückel analysis Īccording to Hückel's rule which states that a cyclic molecule is aromatic if it has (4n + 2) π electrons and antiaromatic if there are 4n electrons, boroles represent antiaromatic molecules.Once reduced to the dianion, the borolediide complex gains aromaticity and can then participate in similar reactions as the Cp − anion, including forming sandwich complexes.
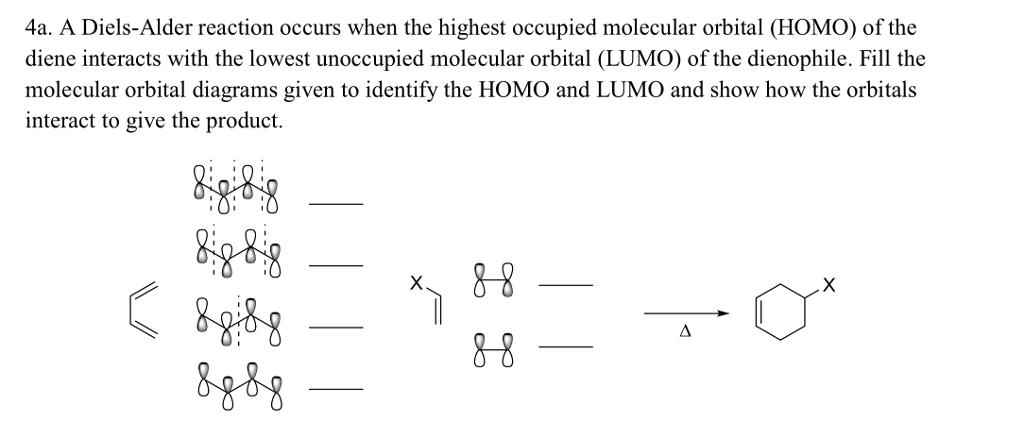
HOMO LUMO DIELS ALDER FREE
The high electron deficiency leads to various reactivities such as metal free hydrogen activation and rearrangements upon cycloaddition which are unobserved in other structural analogues like pyrrole or furan. Substituted derivatives, which have been synthesized, can have various substituents at the 4 carbons and boron. The parent unsubstituted compound with the chemical formula C 4 H 4 BH has yet to be isolated outside a coordination sphere of transition metals. As a result, boroles exhibit unique electronic properties not found in other metalloles.

Although Hückel's rule cannot be strictly applied to borole, it is considered to be antiaromatic due to having 4 π electrons. They are isoelectronic with the cyclopentadienyl cation C 5H + 5(Cp +) and comprise four π electrons. As such, they can be viewed as structural analogs of cyclopentadiene, pyrrole or furan, with boron replacing a carbon, nitrogen and oxygen atom respectively. Sorry.Boroles represent a class of molecules known as metalloles, which are heterocyclic 5-membered rings. The fact that your pyro will be cancelled out by the weakening effect in your auntie pipe, I ogle So this has caused the plane to lose its single bond and go us loose. Your pie ogle the effect that they're going to have the shrink thing. Or this means that this means that the electron, your ante pi orbital will can't love the Elektron. You have one electron in your pie orbital one lunch on your ante pi. It's very difficult to twist in the excited state. See, so in the ground states, you have a double bond, and that's extremely rigid. So party is a CC bond and actually easier twist in the Grand Sea or the excited state. So I would expect the CC bond and M fling to be weaker in the excited state in the ground state. That's going to be that going to you have less electrons, Your pyre world that's going to weaken your bonds and taking Elisa empire a brutal everything your auntie bonding in your ante pi orbital, which by definition makes your bonding less stable, weakens it further. All cheery limo, You know? No, they have less electrons in your pie Orbital. Let's say you take this electron and in you move it to your pie. They wantto Oh, what's the Louima? The limo's going to your Auntie Pie or Paul Soc? Is the cc bun at ling stronger or weaker in the excited state than in the ground state? Why? Well, if you if you take this electron, that's it. Your homo is your highest occupied molecular orbital, your loom and B. Absorption in between door version of a photonic purple waveland can result in the home alumina transition Was the homo in Italy the homo and ethylene? That's going to be your pie orbital? You remember your home. And actually, there's a pair of electrons in the bonding high open up between the two carbons. So the Angie Little diagram and figured nine point six shows that the sideways overlap of a pair of orbital's produces two molecular roubles on bonding. Guys we're doing Problem one twelve, Chapter nine and Chemistry Central Science.


 0 kommentar(er)
0 kommentar(er)
